|
|
|
|
Standard Vulcanization Press |
ECO Vulcanization Press |
Laboratory Vulcanization Press |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vulcanization Press Models
|
|
|
|
| MODEL | Standard Vulcanization Press | ECO Vulcanization Press | Laboratory Vulcanization Press |
| Hydraulic Sliding Table | Standard | --- | --- |
| Middle and Side Extractor Mechanism | Option | Option | --- |
| Hydraulic Oil Cooling Unit | Standard | Standard | --- |
| Ventilation | Standard | Standard | Standard |
| Safety Light Barrier | Standard | Standard | --- |
| Automatic Degassing | Standard | Standard | Standard |
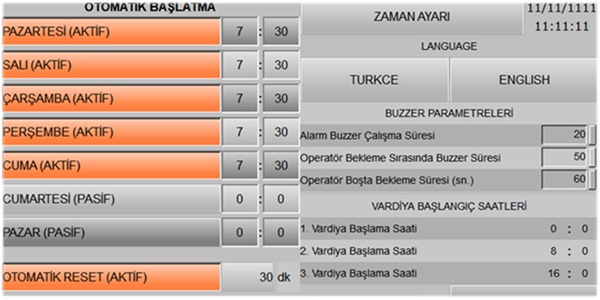
| Automatic Operation and Shutdown | Standard | Standard | --- |
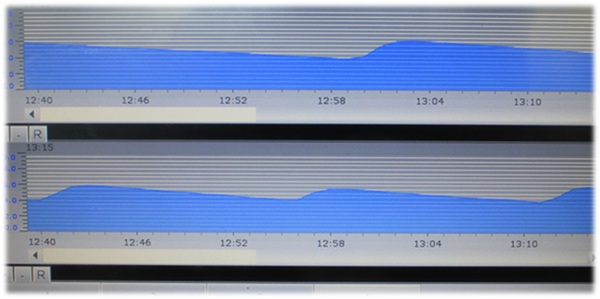
| Electronic Pressure Monitoring | Standard | Standard | Standard |
| Automatic Lubrication | Option | Option | --- |
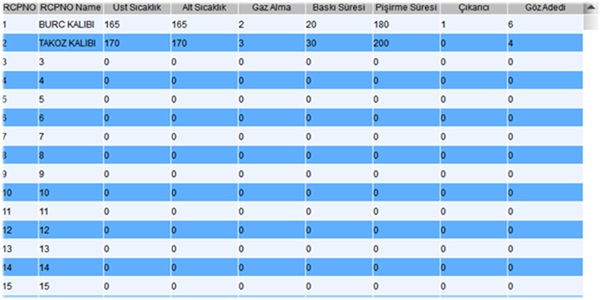
| Mold Parameters Memory | Standard | Standard | --- |
| Operator Delay Warning | Standard | Standard | --- |
| Heating Technology | Standard | Standard | Standard |
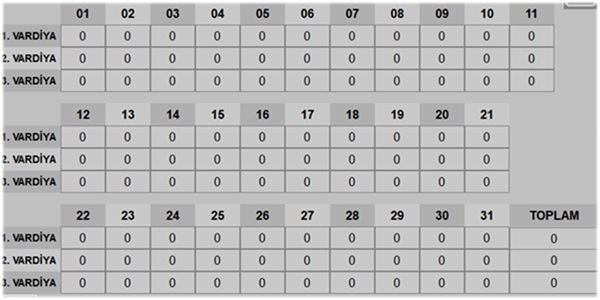
| Shift Production Tracking | Option | Option | --- |
| Direct Mold Heating | Option | Option | --- |
| Server Connection and Remote Control | Option | Option | --- |
| Temperature Statistics of Tables | Standard | Standard | --- |
| Energy Consumption Tracking | Option | Option | --- |
| Multilingual Control Panel | Standard | Standard | --- |
Standard Vulcanization Press |
ECO Vulcanization Press |
Laboratory Vulcanization Press |
|
BROCHURE |
BROCHURE |
BROCHURE |
Yaylacık mh. 44. sk. No:13 16280 Nilüfer / BURSA /TURKEY
+90 224 3611940-41 info@bursamak.com